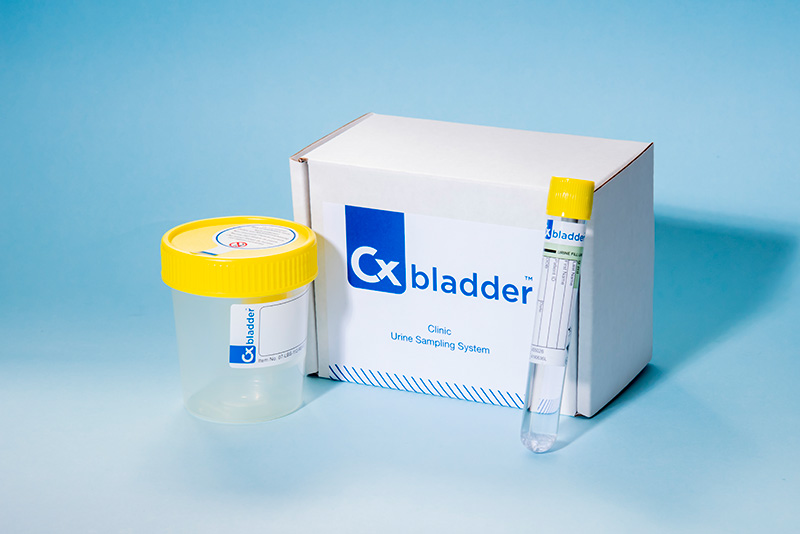
Cxbladder for hematuria evaluation
Cxbladder tests are optimized for the risk stratification of urothelial cancer in patients presenting with microhematuria and help de-intensify clinical work-up in patients with a low probability Cxbladder test result. This reduces the need for referral and invasive procedures, while helping to prioritize those that require immediate care.
Effectiveness of cystoscopy. US data highlights the challenge
The need
Each year over 7 million US patients present with hematuria and 2 million are referred to urology for evaluation. 75% of these patients present with microhematuria.
The challenge
While hematuria is the most common symptom of urothelial cancer, the incidence is low among microhematuria patients and around 95% of those cystoscopies yield a negative result.
The solution
95-97% of patients presenting with microhematuria do not have urothelial cancer and 70-80% of cystoscopies can be safely avoided using a non-invasive Cxbladder test.
Genomic testing designed to optimize practice workflow
Cxbladder Triage
As an established Cxbladder test to support risk stratification, Triage is optimized to help de-intensify clinical work-up in microhematuria patients with a low probability Triage test result, reducing the need for referral and invasive procedures, while prioritizing those who require further investigation.
Cxbladder Triage is included in the AUA/SUFU Microhematuria Guideline, recommended with supporting "Grade A" Evidence**.
Cxbladder Triage Plus
Cxbladder Triage Plus is a next generation Cxbladder test that employs a novel combination of mRNA and DNA biomarkers to extend best in class performance.
Triage Plus enhances risk stratification, reducing unnecessary cystoscopies in patients with a low probability of UC, while prioritizing those who need immediate care.
With analytical and clinical validation shown in published studies*, Triage Plus builds on the performance of Cxbladder Triage, a test now included in the AUA/SUFU Microhematuria Guideline**, supported with "Grade A" evidence from the STRATA randomized controlled trial.***

Featured resource
DRIVE Study validates diagnostic performance of Cxbladder Triage Plus
Leading industry platform Urology Times reviews the DRIVE study, now published in Urologic Oncology. DRIVE provides clinical validation for our next generation Cxbladder test Triage Plus. Cxbladder Triage Plus employs a novel combination of mRNA and RNA biomarkers to extend best in class performance, enhancing the risk stratification of patients presenting with hematuria.
Start your Cxbladder journey
Contact our customer service team
Learn more about the Cxbladder suite and how to implement it in your practice.
Cxbladder Triage
A test optimized to risk stratify and rule out urothelial cancer as a frontline diagnostic
Analyzes gene expression alongside information on known UC risk factors like age, gender and smoking history to help rule out microhematuria patients with a low risk of disease, while prioritizing those who require further investigation.
Safely de-intensifies clinical workup in patients with a low probability Triage test result, while prioritizing those that require immediate care.
Supports a new model for clinical practice and resource utilization. This deployment model provides care to the greatest number of patients and offers clinically actionable information at the earliest point in the patient care pathway.
Included in the AUA/ SUFU Microhematuria Guideline for the management of intermediate risk patients, with supporting "Grade A" Evidence)**.
Featured resources

The Role of Urine-based Biomarkers in Microhematuria Evaluation
In a recent interview with Urology Times, Dr. John Sfakianos discusses the significance of urine-based biomarkers for intermediate-risk patients and outlines key findings from the STRATA randomized controlled trial.

Advancing Microhematuria Evaluation: The Impact of Urinary Biomarkers and Updated Guidelines
Dr. Jay Raman and Dr. Yair Lotan sit down with Dr. Zachary Klaassen to discuss the latest developments in microhematuria evaluation. Topics include the unmet need in risk stratifying patients, the evolution of the AUA/SUFU Microhematuria Guideline from 2020 to 2025, and the role of urinary biomarkers in optimizing patient care, including Cxbladder Triage, a test now included in the Guideline**.

Real-World Implementation of Biomarkers for Microhematuria Workup
Kaiser Permanente's Dr. Ronald Loo and Dr. Christopher Filson join Dr. Zachary Klaassen to discuss the real-world implementation of Cxbladder Triage within the Kaiser Health System for risk stratification, including the results of a published study of over 3,300 patients.

Optimize your clinical practice while improving the patient experience
The Cxbladder suite helps you prioritize your time and clinical resources on those that need it the most, streamlining practice workflow and increasing overall efficiency.
Cxbladder works for both you and the patient. Spare patients with a low probability of urothelial cancer the discomfort, anxiety and risks of an invasive procedure. With urine-based sampling and the option of in-home sample collection, the Cxbladder suite allows you to optimize your clinical practice while delivering the best patient experience possible.
Cxbladder is a precision test that delivers exceptional performance and clinically actionable results
Cxbladder is an objective test with high sensitivity and NPV.
The Cxbladder suite is supported by an evidence portfolio that includes over 30 peer reviewed publications in leading medical journals.
Cxbladder Triage is now included in the AUA/SUFU Microhematuria Guideline with supporting "Grade A" Evidence**.
The Cxbladder suite of tests have been used by over 5,000 urologists in more than 100,000 patients.
How Cxbladder works

The sampling process
Cxbladder's non-invasive urine sampling system is easy for patients to use, streamlining deployment in-clinic.
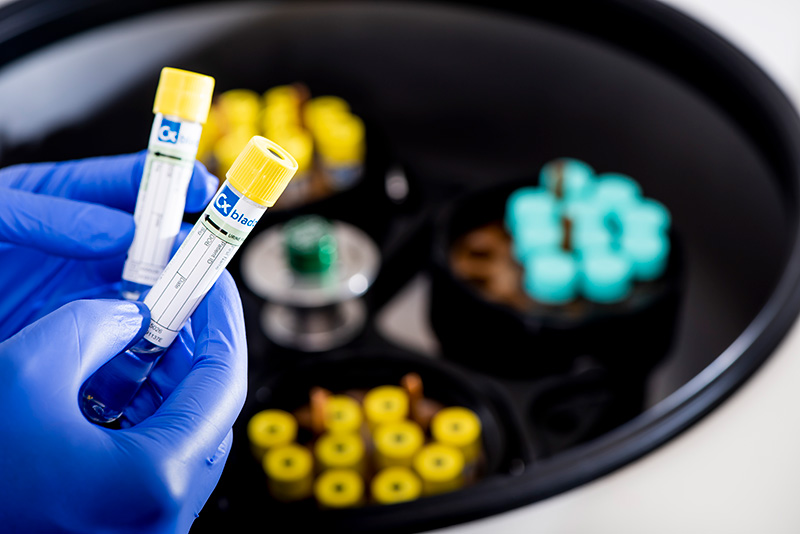
The science
Cxbladder is an objective test with high sensitivity and NPV that delivers exceptional performance and clinically actionable results. The test works at a molecular level, analyzing biomarker genes to help risk stratify urothelial cancer.

The test report
The Cxbladder test report have been developed in consultation with leading urologists and provides the patient’s Cxbladder score, alongside an interpretation of the results and an assay description.

The Cxbladder test report
The Cxbladder test report have been developed in consultation with leading urologists and provides the patient’s Cxbladder score, alongside an interpretation of the results and an assay description.
Start your Cxbladder journey
Contact our customer service team
Learn more about the Cxbladder suite and how to implement it in your practice.
Adopting Cxbladder in your clinic

Implementing Cxbladder
Our team are ready to guide and support you in the deployment and use of Cxbladder. Learn more implementation.

Start your Cxbladder journey
Contact our customer service team to learn more about the Cxbladder suite and how to implement it in your practice.
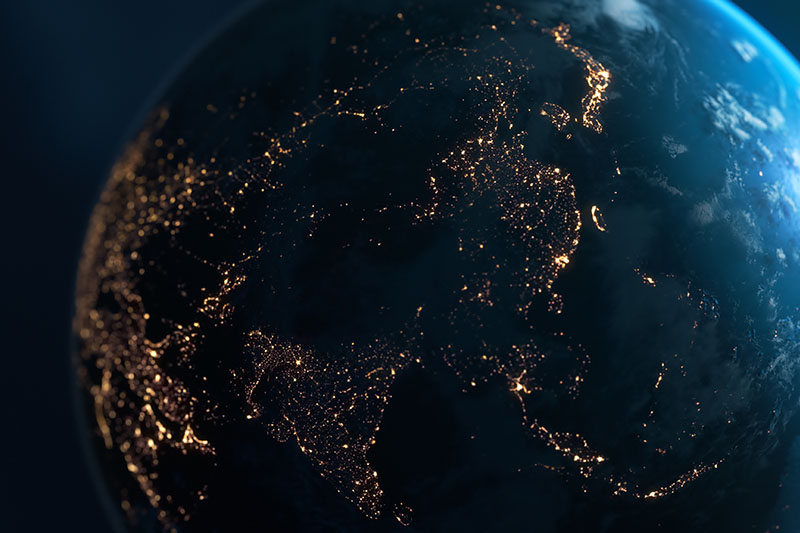
Cxbladder is available around the world
Cxbladder is available in the United States, and in countries across Asia and South America. Cxbladder is also available in New Zealand, Australia, and Israel.
References
* Harvey et al. (2025) Analytical Validation of the Cxbladder® Triage Plus Assay for Risk Stratification of Hematuria Patients for Urothelial Carcinoma. Diagnostics. 2025; 15(14):1739. https://doi.org/10.3390/diagnostics15141739.
* Savage et al. (2025) Diagnostic performance of Cxbladder Triage Plus for the identification and stratification of patients at risk for urothelial carcinoma: The multicenter, prospective, observational DRIVE study. Urologic oncology, S1078-1439(25)00405-3. Advance online publication. https://doi.org/10.1016/j.urolonc.2025.10.008
** Barocas et al. (2025) Updates to Microhematuria: AUA/SUFU Guideline (2025). The Journal of urology, 213(5), 547–557. https://doi.org/10.1097/JU.0000000000004490.
*** Lotan et al. (2024) A Multicenter Prospective Randomized Controlled Trial Comparing Cxbladder Triage to Cystoscopy in Patients With Microhematuria. The Safe Testing of Risk for Asymptomatic Microhematuria Trial. The Journal of Urology Vol 212(1) 41-51.

